Abstract
Background: KMT2a gene rearrangement (KMT2Ar) is a recurrent mutation in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), occurring both in children and adults. Patients with KMT2Ar acute leukemia have poor outcomes related to increased rates of relapsed and refractory disease. Novel therapies including BITE and CAR-T cells has proven efficacious for subgroups of patients with acute leukemia, however those with KMT2Ar are at risk for lineage switch relapse following immunotherapy. In a recent analysis, there have been no reported survivors following lineage switch relapse. Therefore, it is critical to identify novel targets and treatment strategies for KMT2Ar acute leukemia. We have previously demonstrated leukocyte immunoglobulin like receptor B4 (LILRB4) is a novel tumor associated antigen with complete expression on AML with monocytic differentiation (FAB M5), which can be effectively targeted by a novel anti-LILRB4 CAR-T cell we developed and validated in preclinical models. A majority of acute monocytic leukemia cell lines and patient samples contain the KTMT2Ar, which led us to hypothesize LILRB4 is expressed on other KMT2Ar acute leukemias including B-ALL, and may retain expression on lineage switch relapsed disease. In the current study, we evaluate the anti-leukemia function of anti-LILRB4 CAR-T cells against KMT2Ar ALL and lineage switch disease.
Methods and results: We first analyzed the expression of LILRB4 on a panel of B-ALL cell lines by flow cytometry. The KMT2Ar cell lines, RS411 [t(4;11)] and KOPN8 [t(11;19)] demonstrated complete expression of LILRB4 regardless of fusion gene partner, while cell lines without KMT2Ar including 697 and NALM6, were negative for LILRB4. We next analyzed expression of LILRB4 on primary patient samples of de novo, relapsed or refractory acute leukemia identified as KMT2Ar from our institution biospecimen repository. In five cases of B-ALL with KMT2Ar, LILRB4 was co-expressed with CD19 on leukemia blasts as demonstrated by flow cytometry. In one patient with multiply relapsed AML with KMT2Ar, expression of LILRB4 was retained on each relapse following stem cell transplant. Lastly, in an infant with refractory KMTAr B-ALL who developed lineage switch AML following treatment with Blinatumomab, LILRB4 was strongly expressed on the complete population of leukemia blasts.
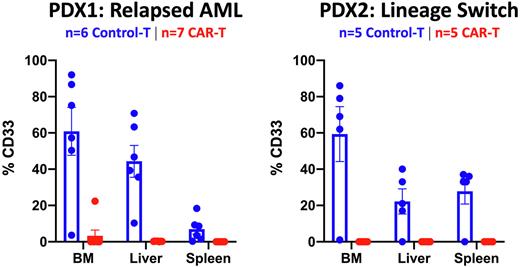
We next evaluated the effector function of anti-LILRB4 CAR-T cells against LILRB4-positive, KMT2Ar B-ALL cell lines. Human T-cells were activated with CD3/CD28 beads, then transduced with lentiviral vector encoding the second generation anti-LILRB4 CAR transgene, incorporating a 4-1BB co-stimulatory and CD3-zeta activation domain, and were expanded for 7-10 days with IL7 and IL15. In vitro, anti-LILRB4 CAR-T cells demonstrated potent cytotoxicity in mixed culture against KOPN8, and similarly displayed significantly increased cytokine release of IL2, TNF-alpha and Interferon-gamma compared to control-T cells. To evaluate the function of anti-LILRB4 CAR-T cell in vivo, we engrafted KOPN8 cells in NSG immunocompromised mice, followed by treatment with control or CAR-T cells. Anti-LILRB4 CAR-T cell treated mice demonstrated significantly decreased leukemia burden and prolonged survival compared to control treated mice. Patient derived mouse xenografts (PDX) of KMT2Ar relapsed AML (PDX1) and lineage switch leukemia (PDX2) were also established. Significantly, when utilizing autologous anti-LILRB4 CAR-T cells, leukemia was eliminated in the PDX1 model. And strikingly in PDX2, we demonstrate elimination of KMT2Ar lineage switch leukemia in mice treated with anti-LILRB4 CAR-T cells.
Conclusion: In this preclinical work, we demonstrate LILRB4 is expressed on KMT2Ar acute leukemias including B-ALL, and importantly displays a retained pattern of expression on fatal lineage switch relapsed AML. Furthermore, we demonstrate potent anti-leukemia activity of anti-LILRB4 CAR-T cells against LILRB4 expressing KMT2Ar acute leukemia, broadening the application of this cell therapy. This work offers a new treatment strategy for patients with dismal outcomes and will be tested in a Phase I clinical trial for children and adults with LILRB4 expressing acute leukemia.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal